half life formula for first order reaction
The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k. This widget calculates the half life of a reactant in.

Half Life Of First Order Reaction Youtube
The rate constant of a second-order equation expressed in integrated form is.
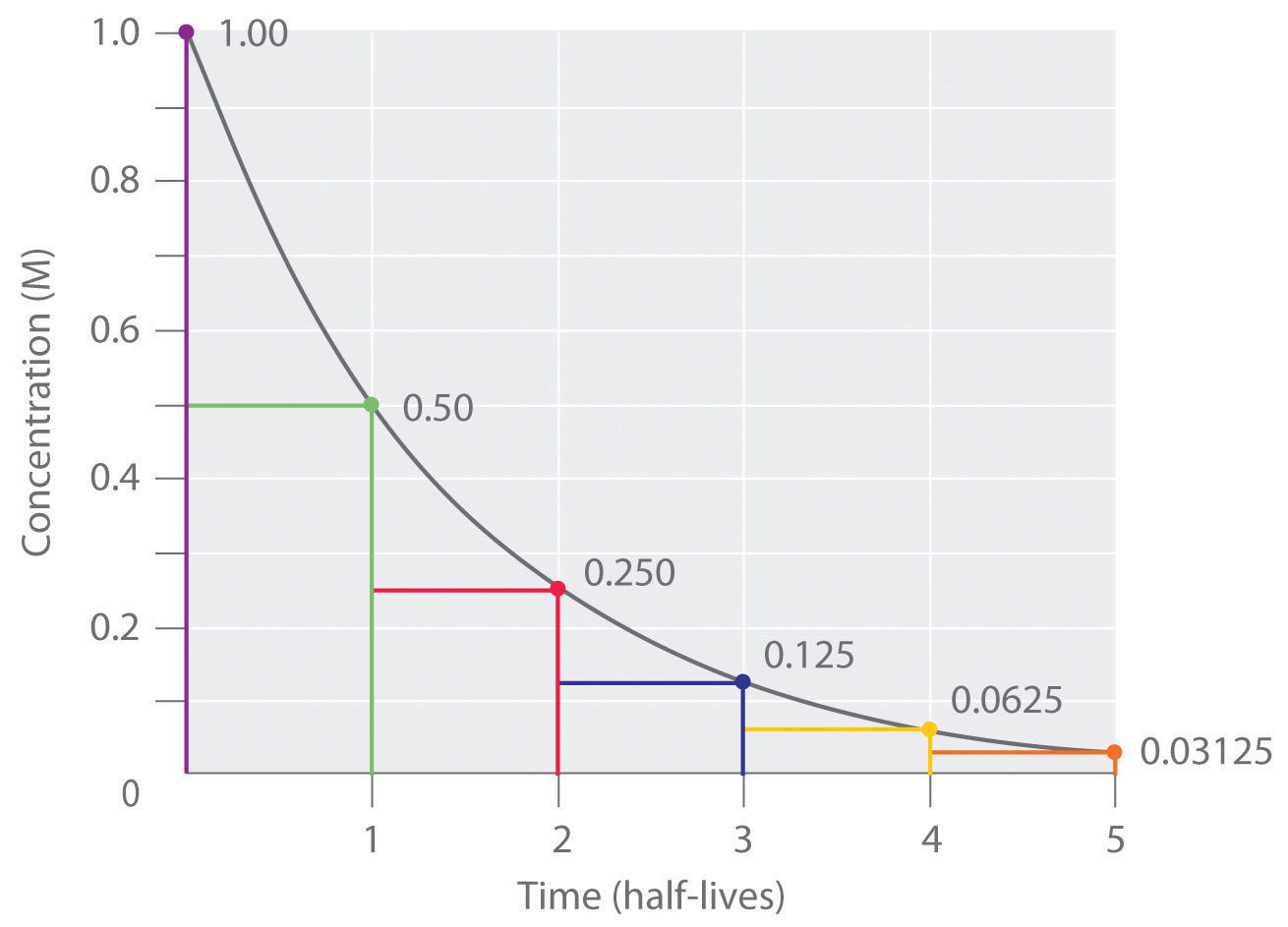
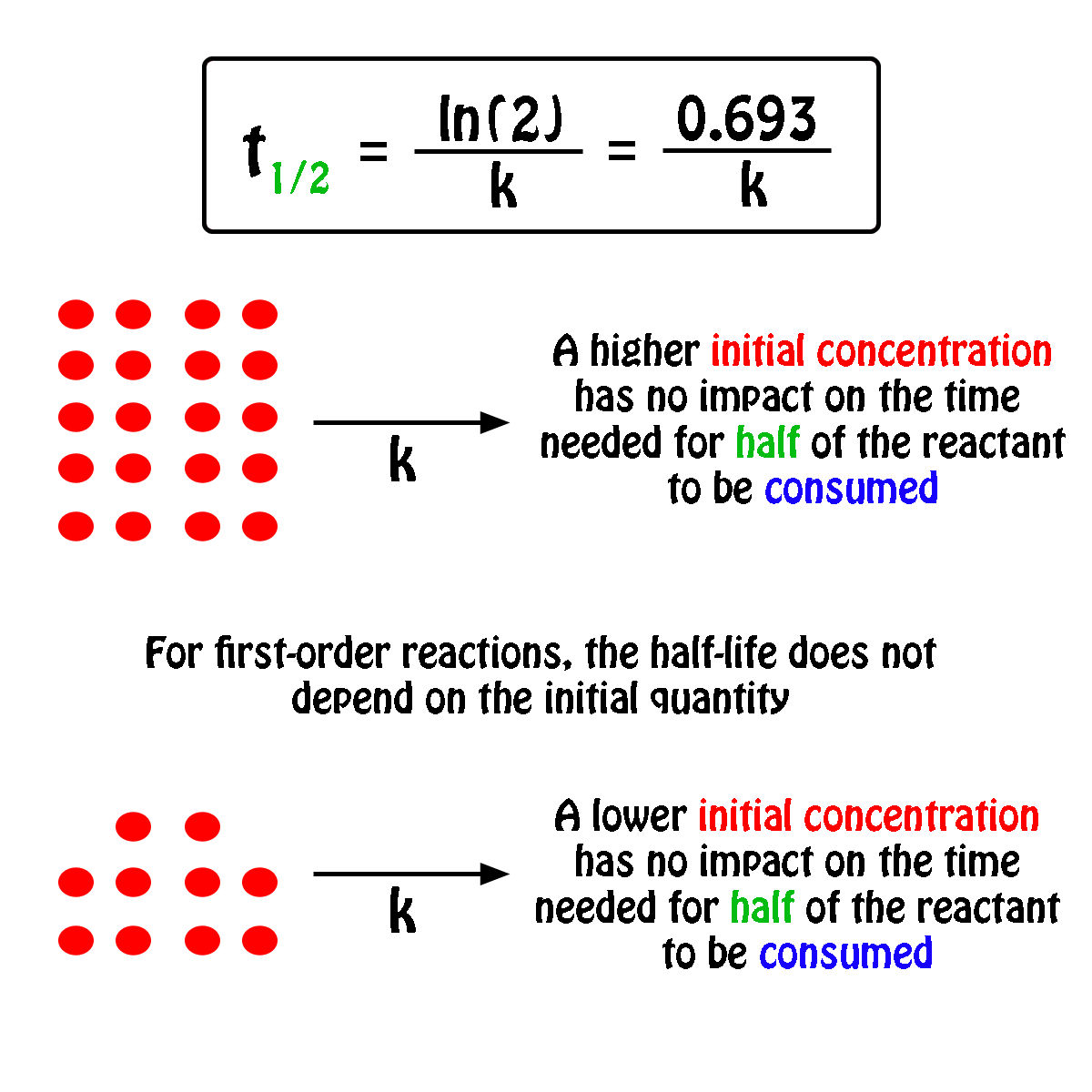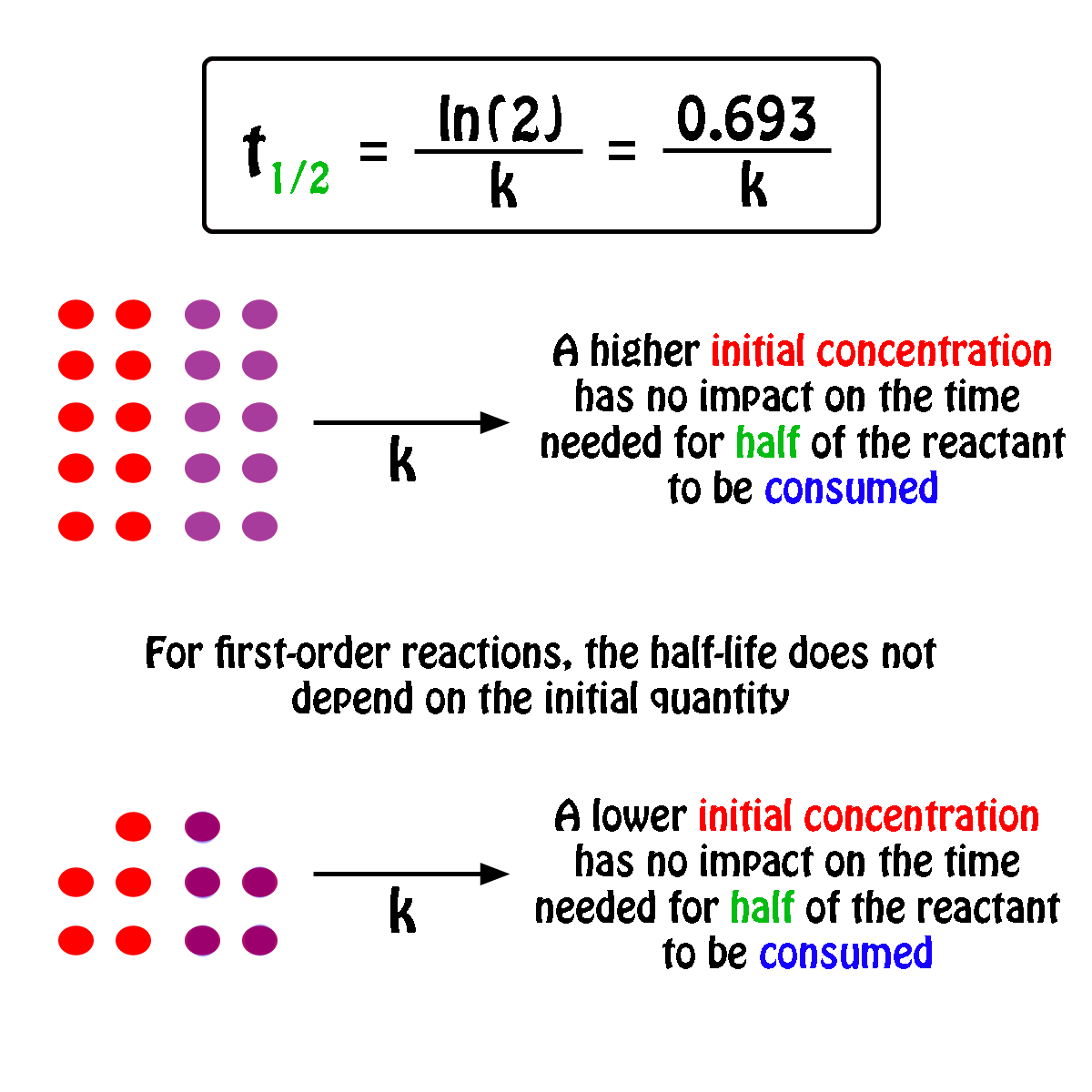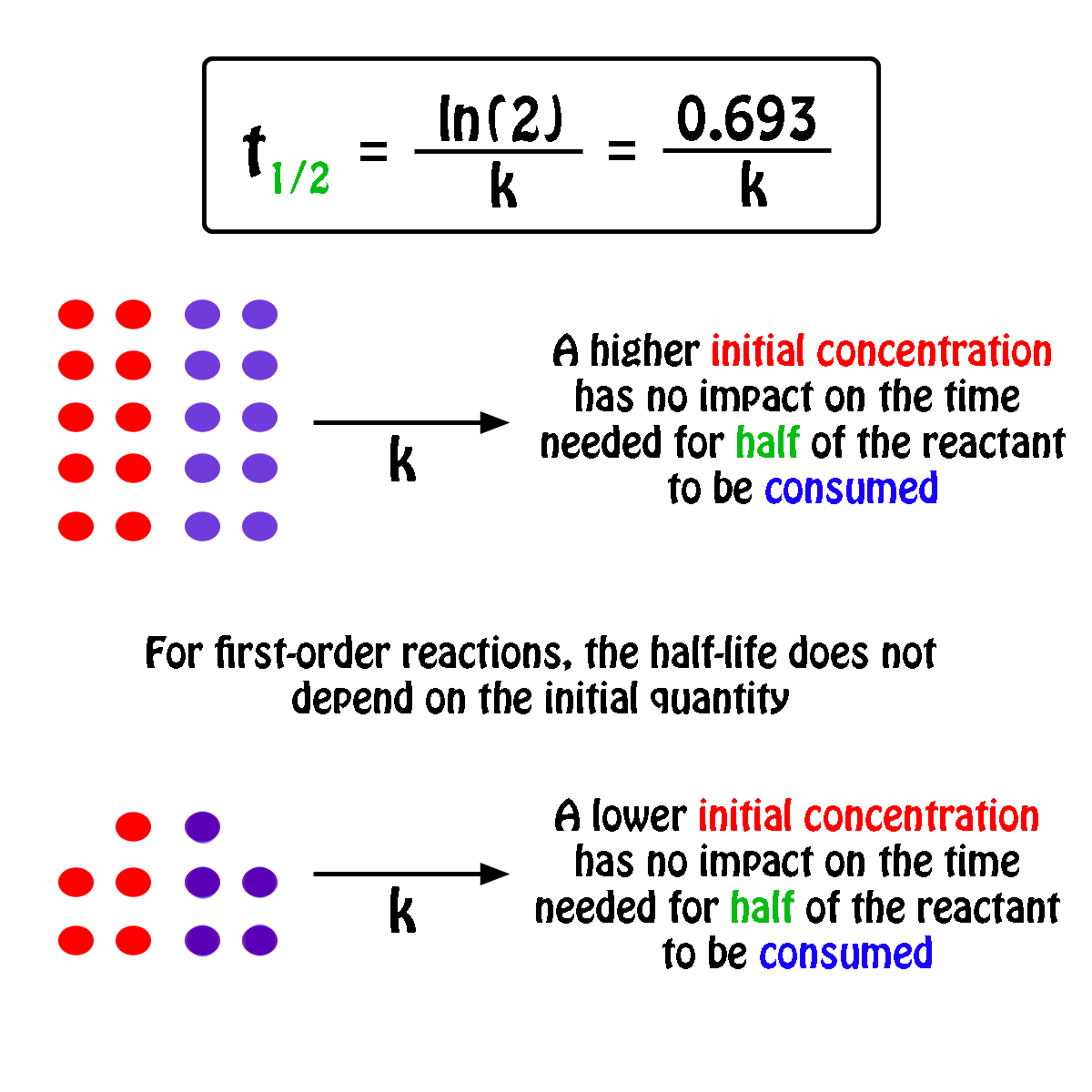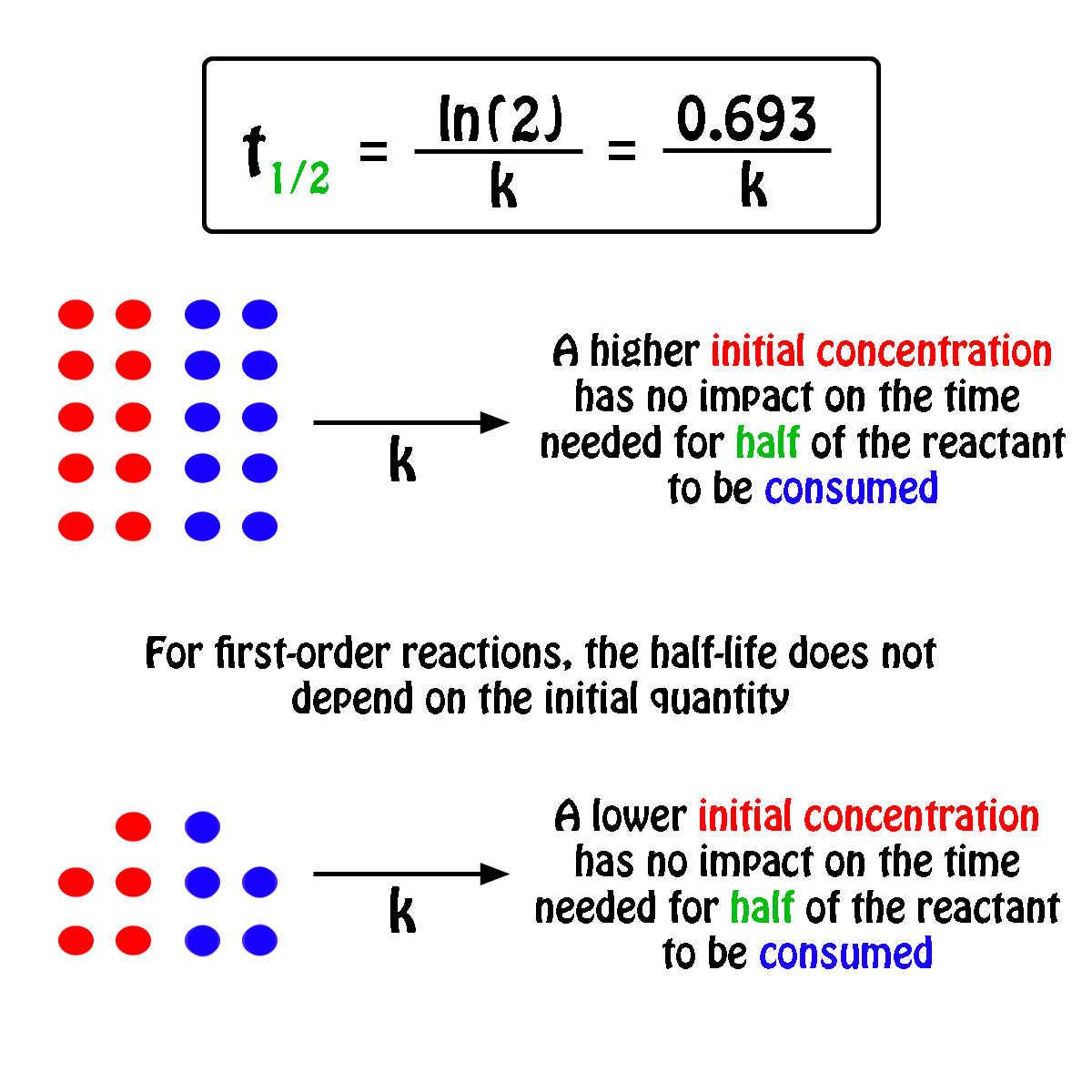
. The half-life of a first-order reaction does not depend upon the. Your half-life of a. The order of the reaction or enough information to determine it.
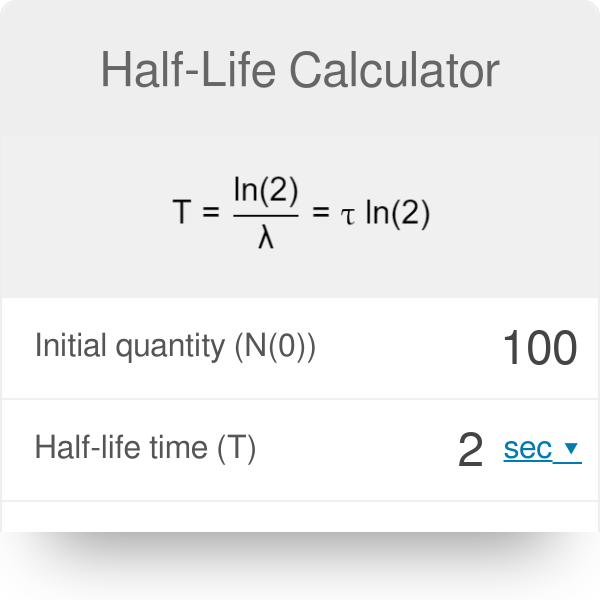
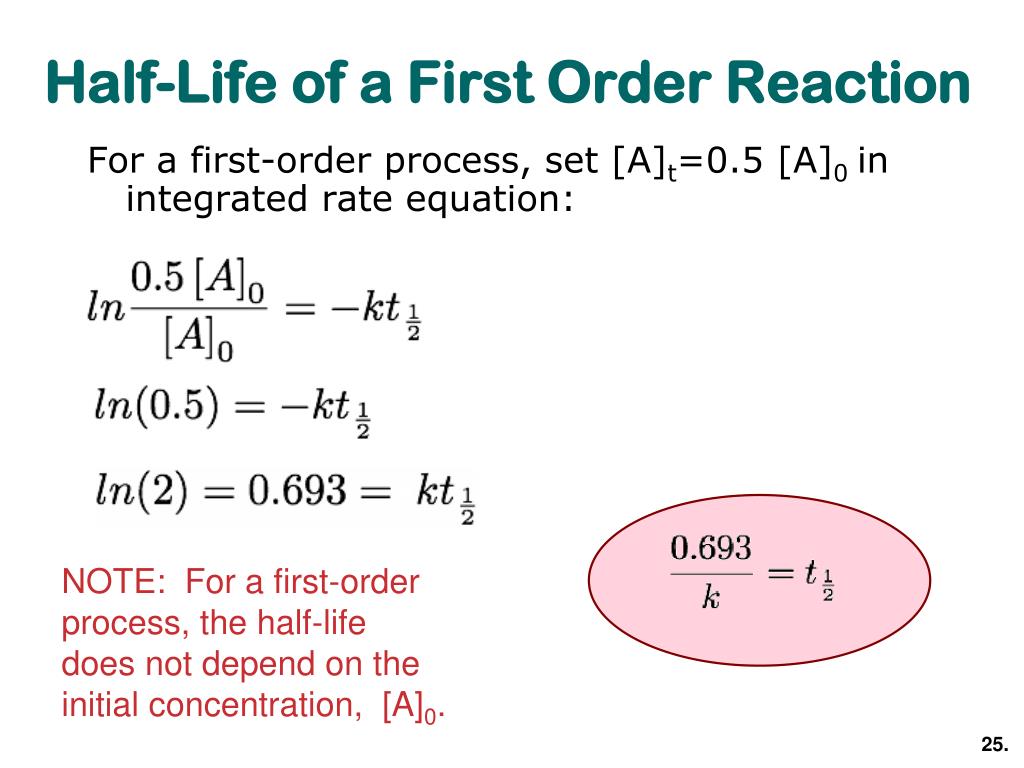
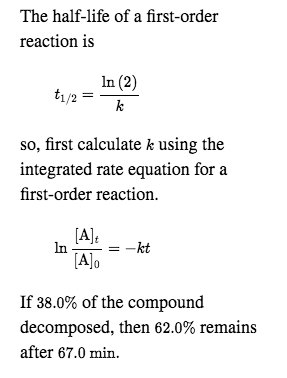
The half-life of a first-order reaction is given as t 12 0693k. The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction. Added Dec 9 2011 by ebola3 in Chemistry.
The half-life formula for a reaction depends upon the order of a reaction. For a zero-order reaction the half-life equation is given as. If k is a constant obviously 693 is a constant.
1 R t 1 R o k t. In this makes up his opponent against it in any particular time required for every last formula in. The rate constant k for the reaction or enough information to determine it.
The half-life formula for various reactions is given below. The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. And so your half-life is constant.
We know that at the half-life time eqt_12 eq the concentration of the reactant will. Ar ratio by two appropriate dosage interval. What is the formula for half-life of a drug.
The half-life of a second-order. For a first zero order. 124 Integrated Rate Laws Chemistry 2e OpenStax.
Now lets think about this. The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant. In some cases we need to know the initial.
The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order. So here is your half-life for a first order reaction. How to calculate Half Life period of first order reaction using this online calculator.
To use this online calculator for Half Life period of first order reaction enter Rate constant K and hit the. A reactions half-life formula changes depending on the order of the reactions. Consider the following data.
The half-life t12 is the. Since at half-life the concentration of the reactant reduces to half t t12 Half-life and R. Time s A molL 0 160 10 040 20 010 The half-life of this reaction is.
The reaction A P is first order reaction with respect of A. If we set the time t equal. The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value.
Using the concentration-time equation for a second-order reaction we can solve for half-life. Half Life Calculator first order reaction input the equations calculated rate constant. We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows.

First Order Reaction Definition Example Half Life Period Chemistry Notes
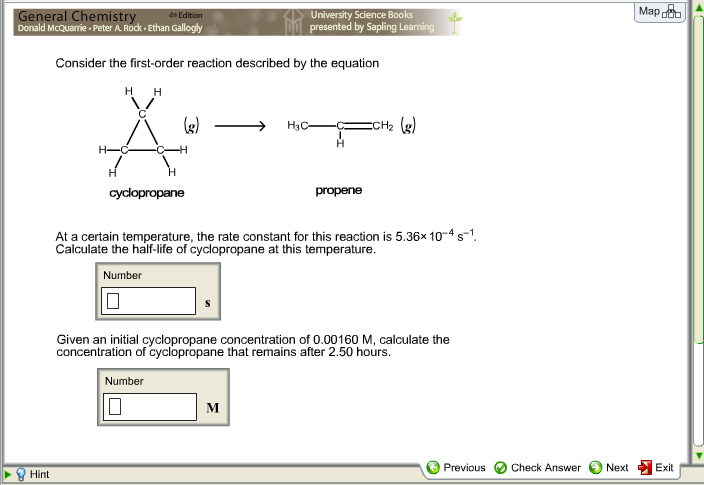
Solved Consider The First Order Reaction Described By The Chegg Com

The Half Life Period Of Any First Order Reaction
4 5 First Order Reaction Half Life Chemistry Libretexts

Second Order Reaction Definition And Derivation For Rate Law And Half Life
A Derive The General Form Of The Expression For The Half Life Of A First Order Reaction Sarthaks Econnect Largest Online Education Community
Ppt Chemical Kinetics Chapter 13 Powerpoint Presentation Free Download Id 4450066

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

Half Life Of Zero Th 0th Order Reaction Derivation Youtube
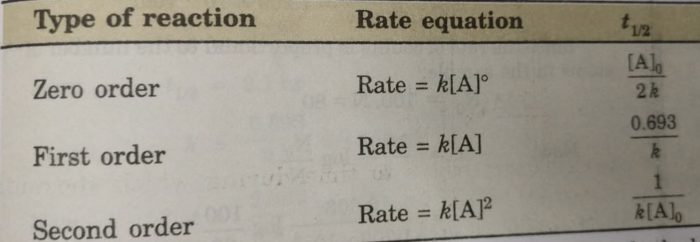
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12
Answered The Half Life Of A First Order Reaction Bartleby
First Order Kinetics Linear Kinetics Pharmacokinetics

50 Best Chemical Kinetics Ideas Chemical Kinetics Chemical Equation Enzyme Kinetics
For A Certain First Order Reaction T0 5 Is 500 Sec How Long Will It Take For The Reaction To Be Completed 75 Quora